library(kableExtra) # for creating tables
options(kableExtra.html.bsTable = T)
library(dplyr) # for data wrangling
library(broom) # for pulling off coefficients and SEs from lm
library(ggplot2) # for plotting8 Modeling Strategies
Learning objectives
Gain an appreciation for challenges associated with selecting among competing models and performing multi-model inference.
Understand common approaches used to select a model (e.g., stepwise selection using p-values, AIC, Adjusted
Understand the implications of model selection for statistical inference.
Gain exposure to alternatives to traditional model selection, including full model inference (df spending), model averaging, and penalized likelihood/regularization techniques.
Be able to evaluate model performance using cross-validation and model stability using the bootstrap.
Be able to choose an appropriate modeling strategy, depending on the goal of the analysis (describe, predict, or infer).
8.1 R Packages
We begin by loading a few packages upfront:
In addition, we will use data and functions from the following packages:
openintrofor themammalsdata setMASSfor thestepAICfunctionabefor augmented backwards selectionMuMinfor model averaging based on AIC weightsglmnetfor an implementation of the LASSO algorithmcaretfor performing cross-validationrmsfor evaluating models using the bootstrap
8.2 Goals of multivariable regression modeling
Before deciding on an appropriate modeling strategy, it is important to recognize that there are multiple possible objectives motivating the need for a model (Shmueli 2010; Kuiper and Sklar 2012; Tredennick et al. 2021). Kuiper and Sklar (2012) lists 3 general modeling objectives, to describe, predict, or explain, which closely mimics the goals outlined by Tredennick et al. (2021), exploration, prediction, and inference.
Describe: we may just want to capture the main patterns in the data in a parsimonious way. This may be a first step in trying to understand which factors are related to the response variable. Note, describing associations between variables is a much less lofty goal than evaluating cause and effect.
Predict: we may want to use data we have in hand to make predictions about future data. Here, we may not need to worry about causality and confounding (capturing associations may be just fine). We may be able to predict the average life expectancy for a country based on TVs per capita (Figure 7.1) or the number of severe sunburns from local ice creme sales (Figure 7.3). Yet these predictions do not capture causal mechanisms, and thus, we will often find that our model fails to predict when we try to apply it to a new situation where the underlying correlations among our predictor variables differs from the correlations among predictors in the data set we used to fit the model.
Explain/Infer: we may have a biological hypothesis or set of competing hypotheses about how the world works. These hypotheses might suggest that certain variables, or combination of variables, are causally related to the response variable. When trying to infer causal relationships between predictor and response variables, we have to think more broadly about links among our observed predictor variables (not just links between predictors and the response). In addition, we must consider possible confounding by unmeasured or omitted variables. Essentially, we need to keep in mind everything from Chapter 7.
Clearly, the least lofty goal is to explore or describe patterns, but whether one views prediction or inference as the most challenging or lofty goal may depend on whether predictions are expected to match observations in novel situations or just under conditions similar to those used to train the model. If we want to be able to predict what will happen when the system changes, we will need to have a strong understanding of mechanisms driving observed patterns.
It is important to consider your goals when deciding upon a modeling strategy Table 8.1. I collaborate mainly with scientists that work with observational data. Often, they express their questions in broad terms, such as, “I want to know which predictor variables are most important.” When pressed, they may indicate they are interested a bit in all of the above (describing patterns, explaining patterns, and predicting outcomes). The main challenge to “doing all of the above” is that it is all too easy to detect patterns in data that do not represent causal mechanisms, leading to models that fail to predict new data well. This can result in a tremendous waste of money and resources. We need to consider the impact of applying an overly flexible modeling strategy if our goal is to identify important associations among variables (explain) or if our goal is to develop a predictive model (predict). I learned of the dangers of overfitting the hard way while getting my Masters degree in Biostatistics at the University of North Carolina-Chapel Hill (see Section 8.3).
| Parameter | Exploration | Inference | Prediction |
|---|---|---|---|
| Purpose | generate hypotheses | test hypotheses | forecast the future accurately |
| Priority | thoroughness | avoid false positives | minimize error |
| A priori hypothesis | not necessary | essential | not necessary, but may inform model specification |
| Emphasis on model selection | important | minimal | important |
| Key statistical tools | any | null hypothesis significance tests | AIC; regularization; machine learning; cross-validation; out-of-sample validation |
| Pitfalls | fooling yourself with overfitted models with spurious covariate effects | misrepresenting exploratory tests as tests of a priori hypotheses | failure to rigorously validate prediction accuracy with independent data |
If we have competing models for how the world works (e.g., different causal networks; Chapter 7), then we can formulate mechanistic models that represent these competing hypotheses. In some cases you may have to write your own code to fit models using Maximum Likelihood (Chapter 10) and Bayesian machinery (Chapter 11); we will be learning about these methods soon. We can then compare predictions from these models or evaluate their ability to match qualitative patterns in data (“goodness-of-fit”), ideally across multiple study systems, to determine if our theories hold up to the scrutiny of data. If you find yourself in this situation (i.e., doing inference), consider yourself lucky (or good); you are doing exciting science.
8.3 My experience
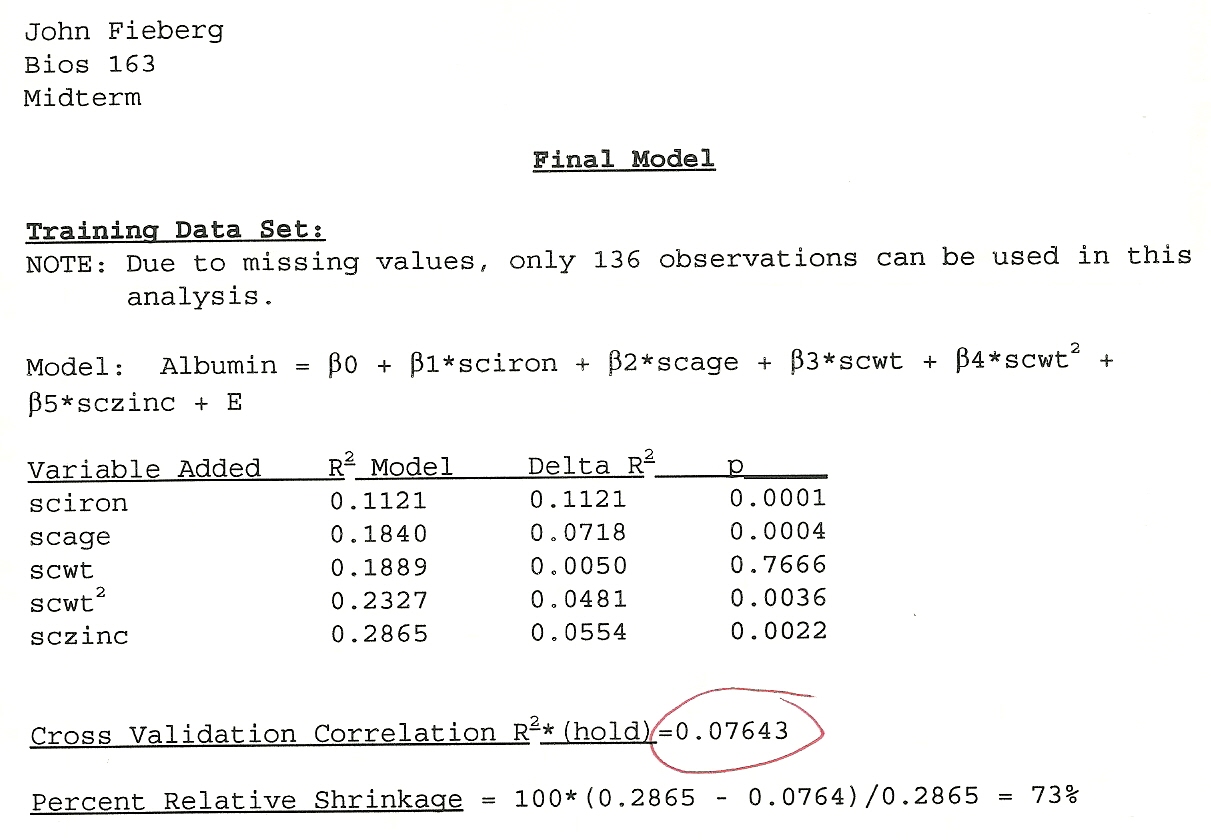
In the first year of my Masters degree, I took a Linear Models class from Dr. Keith Muller. For our midterm exam, we were given a real data set and asked to develop a model for patients’ Serum Albumin levels1. The data set contained numerous predictors, some with missing data. We were given a weekend to complete the analysis and write up a report on our findings. Importantly, we were instructed to take one of two approaches:
If we knew the subject area (or, could quickly get up speed), then we could choose to test a set of a priori hypotheses regarding whether specific explanatory variables were associated with Serum Albumin levels. We were encouraged to consider potential issues related to multiple comparisons and to adjust our
Split the data into a “training” data set (with 80% of the original observations) and a “test” data set (with the remaining 20% of the observations). Use the training data set to determine an appropriate model. Then, evaluate the model’s performance using the test data set. When determining an appropriate model, we were instructed to use various residual and diagnostic plots and to also consider the impact that an overly data-driven or flexible modeling approach might have on future model performance.
I don’t think anyone from our class knew anything about blood chemistry or kidney failure, so I suspect everyone went with option 2. A few things I recall from my approach and the final outcome:
I grouped variables into similar categories (e.g., socio-economic status, stature [height, weight, etc], dietary intake variables) and examined variables within each group for collinearity. I then picked a single “best” variable in each category using simple linear regressions (i.e., fitting models with a single predictor to determine which variable within each group was most highly correlated with the response).
I fit a model that combined the “best” variables from each category and evaluated model assumptions (Normality, constant variance, linearity). Residual plots all looked good - no major assumption violations.
I allowed for a non-linear effect of weight using a quadratic polynomial (see Section 4.3). To be honest, I cannot remember why. The decision, however, was likely arrived at after looking at the data and noticing a trend in the residuals or finding that a quadratic term was highly significant when included in the model.
I applied various stepwise regression methods (see Section 8.4.1) and always arrived at the same reduced model.
I looked to see if I might have left off any important variables by adding them to this reduced model arrived at in step [4]. None of the added variables were statistically significant. This step gave me further confidence that I had found the best model for the job.
Lastly, I again looked at residual diagnostics associated with the reduced model. Everything looked hunky-dory.
I remember being highly confident that I found THE BEST model and that the assumptions were all reasonable. I had such good feelings about how things went initially, that when reflecting back on the assignment many years later, I was sure that my model had an
8.4 Stepwise selection algorithms
There are many ways to sift through model space to determine a set of predictors that are most highly associated with a response variable. At the extreme, one can specify a set of predictors and then fit “all possible models” that include 1 or more of these predictors along with a null model containing only an intercept (e.g., using the dredge function in the MuMIn package; Barton 2020). Having fit all possible models, one must specify a criterion for choosing a “best model.” For linear models,
Alternatively, one can try to successfully build bigger and better models (by adding 1 variable at a time), referred to as forward-stepwise selection, or try to build slimmer and more parsimonious models (by eliminating 1 variable at a time from an initial full model), referred to as backwards elimination. Either approach will result in many comparisons of nested models; two models are nested if you can get from the more complex model to the simpler model by setting one or more parameters equal to 0. As one example, the following two models are nested (you can get from the first model to the second by setting
whereas the two models below are not:
For nested models, we can use p-values from t-tests, F-tests, or likelihood-ratio tests to compare models (in addition to AIC or adjusted-
8.4.1 Backwards elimination and forward-stepwise selection
To apply backwards stepwise selection, we:
- Fit a full model containing all predictors of interest.
- Consider all possible models formed by dropping 1 of these predictors
- Keep the current model, or drop the “worst” predictor depending on:
- p-values from the individual t-tests (drop the variable with the highest p-value if it is greater than some threshold value, not necessarily
- Adjusted
- AIC (lower values are better)
- p-values from the individual t-tests (drop the variable with the highest p-value if it is greater than some threshold value, not necessarily
- Rinse and repeat until you can no longer improve the model by dropping a predictor
The stepAIC function in the MASS library will do this for us. Let’s explore this approach using the mammal sleep data set from Chapter 6. Before we begin, it is important to recognize that this data set has many missing values for several of the variables. This can create issues when comparing models since R will by default drop any observations where one or more of the variables are missing. Thus, the number observations will change depending on which predictors are included in the model, making comparisons using AIC or adjusted
library(openintro)
data(mammals, package="openintro")
mammalsc<-mammals %>% filter(complete.cases(.))
MASS::stepAIC(lm(total_sleep ~ life_span + gestation + log(brain_wt) +
log(body_wt) + predation + exposure + danger, data=mammalsc))Start: AIC=100.44
total_sleep ~ life_span + gestation + log(brain_wt) + log(body_wt) +
predation + exposure + danger
Df Sum of Sq RSS AIC
- log(body_wt) 1 0.412 313.99 98.491
- life_span 1 1.218 314.79 98.598
- log(brain_wt) 1 12.516 326.09 100.079
- gestation 1 13.389 326.96 100.192
<none> 313.58 100.436
- exposure 1 18.527 332.10 100.847
- predation 1 31.079 344.65 102.405
- danger 1 92.636 406.21 109.307
Step: AIC=98.49
total_sleep ~ life_span + gestation + log(brain_wt) + predation +
exposure + danger
Df Sum of Sq RSS AIC
- life_span 1 0.977 314.96 96.621
- gestation 1 13.113 327.10 98.209
<none> 313.99 98.491
- exposure 1 19.446 333.43 99.014
- predation 1 30.876 344.86 100.430
- log(brain_wt) 1 31.497 345.48 100.506
- danger 1 92.380 406.37 107.323
Step: AIC=96.62
total_sleep ~ gestation + log(brain_wt) + predation + exposure +
danger
Df Sum of Sq RSS AIC
- gestation 1 12.206 327.17 96.218
<none> 314.96 96.621
- exposure 1 18.987 333.95 97.080
- predation 1 30.083 345.05 98.453
- log(brain_wt) 1 34.465 349.43 98.983
- danger 1 92.153 407.12 105.400
Step: AIC=96.22
total_sleep ~ log(brain_wt) + predation + exposure + danger
Df Sum of Sq RSS AIC
<none> 327.17 96.218
- exposure 1 16.239 343.41 96.253
- predation 1 41.155 368.32 99.194
- log(brain_wt) 1 90.786 417.96 104.504
- danger 1 108.584 435.75 106.255
Call:
lm(formula = total_sleep ~ log(brain_wt) + predation + exposure +
danger, data = mammalsc)
Coefficients:
(Intercept) log(brain_wt) predation exposure danger
16.5878 -0.8800 2.2321 0.9066 -4.5425 We see a set of tables comparing the current “best” model (<none> indicating no variables have been dropped) to all possible models that have 1 fewer predictor, sorted from lowest (best fitting) to highest (worst fitting) as judged by AIC. For example, in the first table, we see that dropping any of (log(body_wt), life_span, log(brain_wt), gestation) will result in a model with a lower AIC than the full model containing all variables. On the other hand, dropping any of (exposure, predation, or danger) will result in a model with a worse AIC than the one associated with the full model. At this step, we drop log(body_wt) since dropping it results in a model with the lowest AIC (98.491) among all 6-variable models. We repeat the algorithm, dropping lifespan, and then once more, dropping gestation. At that point, dropping any of (log(brain_wt), predation, exposure, danger) will result in a model that has a higher AIC, so we stop.
Forward stepwise selection moves in the opposite direction and can be implemented using the step function:
min.model <- lm(total_sleep ~ 1, data=mammalsc)
step(min.model, scope=( ~ life_span + gestation + log(brain_wt) + log(body_wt) +
predation + exposure + danger),
direction="forward", data=mammalsc)Start: AIC=131.15
total_sleep ~ 1
Df Sum of Sq RSS AIC
+ log(brain_wt) 1 352.15 557.17 112.58
+ exposure 1 351.08 558.25 112.66
+ log(body_wt) 1 346.71 562.62 112.99
+ gestation 1 343.34 565.98 113.24
+ danger 1 332.07 577.25 114.07
+ predation 1 148.94 760.38 125.64
+ life_span 1 133.00 776.32 126.51
<none> 909.32 131.15
Step: AIC=112.58
total_sleep ~ log(brain_wt)
Df Sum of Sq RSS AIC
+ danger 1 179.991 377.18 98.192
+ predation 1 118.745 438.43 104.512
+ exposure 1 83.765 473.41 107.736
+ gestation 1 38.295 518.88 111.588
<none> 557.17 112.579
+ life_span 1 11.296 545.88 113.718
+ log(body_wt) 1 6.190 550.98 114.109
Step: AIC=98.19
total_sleep ~ log(brain_wt) + danger
Df Sum of Sq RSS AIC
+ predation 1 33.773 343.41 96.253
+ gestation 1 19.139 358.04 98.005
<none> 377.18 98.192
+ exposure 1 8.857 368.32 99.194
+ life_span 1 0.519 376.66 100.135
+ log(body_wt) 1 0.354 376.83 100.153
Step: AIC=96.25
total_sleep ~ log(brain_wt) + danger + predation
Df Sum of Sq RSS AIC
+ exposure 1 16.2392 327.17 96.218
<none> 343.41 96.253
+ gestation 1 9.4578 333.95 97.080
+ log(body_wt) 1 0.6927 342.72 98.168
+ life_span 1 0.0103 343.40 98.251
Step: AIC=96.22
total_sleep ~ log(brain_wt) + danger + predation + exposure
Df Sum of Sq RSS AIC
<none> 327.17 96.218
+ gestation 1 12.2056 314.96 96.621
+ log(body_wt) 1 0.0943 327.08 98.206
+ life_span 1 0.0696 327.10 98.209
Call:
lm(formula = total_sleep ~ log(brain_wt) + danger + predation +
exposure, data = mammalsc)
Coefficients:
(Intercept) log(brain_wt) danger predation exposure
16.5878 -0.8800 -4.5425 2.2321 0.9066 In this case, we end up at the same place. This may seem reassuring as it tells us that we arrive at the same “best model” regardless of which approach we choose. Yet, what really matters is how these algorithms perform across multiple data sets (see Section 8.8.2). Therein lies many problems. As noted by Frank Harrell in his Regression Modeling Strategies book (Harrell Jr 2015), with stepwise selection:
- The ordinary F and
- SEs of regression coefficients will be biased low and confidence intervals will be too narrow.
- p-values will be too small and do not have the proper meaning (due to multiple comparison issues).
- Regression coefficients will be biased high in absolute magnitude.
- Rather than solve issues with collinearity, collinearity makes variable selection arbitrary.
- Stepwise selection does not require that we think hard about our underlying problem/study system.
Issues with p-values are rather easy to understand in the context of multiple comparisons and multiple hypothesis tests, whereas the bias in regression coefficients may be more surprising to some readers. To understand why regression coefficient estimators will be biased high, it is important to recognize that inclusion in the final model depends not on the true relationship between the explanatory and response variables but rather on the estimated relationship determined from the original sample. A variable is more likely to be included if by chance its importance in the original sample was overestimated than if it was underestimated (Copas and Long 1991). These issues are more pronounced when applied to small data sets with lots of predictors; stepwise selection methods can easily select noise variables rather than ones that are truly important.
Issues with stepwise selection are well known to statisticians, who agree that these methods should be abandoned (Anderson and Burnham 2004; Whittingham et al. 2006; Hegyi and Garamszegi 2011; Giudice, Fieberg, and Lenarz 2012; Fieberg and Johnson 2015). Yet, they are still routinely used, likely because they appear to be objective and “let the data speak” rather than forcing the analyst to think. Stepwise selection methods can sometimes be useful for identifying important associations, but any results should be treated cautiously. I.e., it is best to consider these methods as useful for generating new hypotheses; these hypotheses should be tested with new, independent data.
If you are not convinced, try this simple simulation example posted by Florian Hartig here, that considers a response variable that is unrelated to 100 different simulated predictor variables. On average, we would expect to see
set.seed(1)
library(MASS)
# Generate 200 observations with 100 predictors that are unrelated to the response variable
dat <- data.frame(matrix(runif(20000), ncol = 100))
dat$y <- rnorm(200)And, if we fit a full model that contains all predictors and look at the p-values for the individual hypothesis tests, we find only 2 that have p-values less than 0.05
# Fit a full model containing all predictors and test for significance.
fullModel <- lm(y ~ . , data = dat)
summary(fullModel)
Call:
lm(formula = y ~ ., data = dat)
Residuals:
Min 1Q Median 3Q Max
-1.95280 -0.39983 -0.01572 0.46104 1.61967
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 2.19356 1.44518 1.518 0.1322
X1 0.58079 0.32689 1.777 0.0787 .
X2 -0.52687 0.32701 -1.611 0.1103
X3 0.27721 0.33117 0.837 0.4046
X4 -0.18342 0.30443 -0.602 0.5482
X5 -0.18544 0.29011 -0.639 0.5242
X6 -0.18382 0.31406 -0.585 0.5597
X7 -0.46290 0.28349 -1.633 0.1057
X8 -0.21527 0.29856 -0.721 0.4726
X9 0.12216 0.30359 0.402 0.6883
X10 -0.02594 0.33828 -0.077 0.9390
X11 0.25669 0.29482 0.871 0.3860
X12 -0.10183 0.30164 -0.338 0.7364
X13 0.49507 0.33438 1.481 0.1419
X14 0.16642 0.33659 0.494 0.6221
X15 0.11402 0.32964 0.346 0.7302
X16 -0.17640 0.31619 -0.558 0.5782
X17 -0.03129 0.31830 -0.098 0.9219
X18 -0.28201 0.29681 -0.950 0.3444
X19 0.02209 0.29664 0.074 0.9408
X20 0.25063 0.29855 0.839 0.4032
X21 -0.02479 0.30556 -0.081 0.9355
X22 -0.01187 0.31265 -0.038 0.9698
X23 -0.58731 0.31491 -1.865 0.0651 .
X24 -0.27343 0.32894 -0.831 0.4078
X25 -0.22745 0.29223 -0.778 0.4382
X26 0.18606 0.35755 0.520 0.6040
X27 -0.26998 0.33302 -0.811 0.4195
X28 0.09683 0.32235 0.300 0.7645
X29 0.36746 0.32915 1.116 0.2670
X30 -0.26027 0.31335 -0.831 0.4082
X31 -0.07890 0.28822 -0.274 0.7849
X32 -0.07879 0.32662 -0.241 0.8099
X33 -0.27736 0.34542 -0.803 0.4239
X34 -0.21118 0.34514 -0.612 0.5420
X35 0.17595 0.30706 0.573 0.5679
X36 0.17084 0.30423 0.562 0.5757
X37 0.28246 0.29520 0.957 0.3410
X38 0.01765 0.32873 0.054 0.9573
X39 0.07598 0.27484 0.276 0.7828
X40 0.09714 0.34733 0.280 0.7803
X41 -0.16985 0.31608 -0.537 0.5922
X42 -0.25184 0.33203 -0.758 0.4500
X43 -0.08306 0.29306 -0.283 0.7774
X44 -0.17389 0.31090 -0.559 0.5772
X45 -0.30756 0.30995 -0.992 0.3235
X46 0.61520 0.30961 1.987 0.0497 *
X47 -0.61994 0.32461 -1.910 0.0591 .
X48 0.62326 0.33822 1.843 0.0684 .
X49 0.35504 0.30382 1.169 0.2454
X50 0.09683 0.31925 0.303 0.7623
X51 0.17292 0.30770 0.562 0.5754
X52 -0.06560 0.30549 -0.215 0.8304
X53 -0.29953 0.32318 -0.927 0.3563
X54 0.06888 0.32289 0.213 0.8315
X55 0.05695 0.32103 0.177 0.8596
X56 0.26284 0.32914 0.799 0.4265
X57 0.10457 0.29788 0.351 0.7263
X58 -0.19239 0.30729 -0.626 0.5327
X59 0.02371 0.29171 0.081 0.9354
X60 -0.12842 0.32321 -0.397 0.6920
X61 0.06931 0.30015 0.231 0.8179
X62 -0.27227 0.31918 -0.853 0.3957
X63 -0.17359 0.32287 -0.538 0.5920
X64 -0.41846 0.33808 -1.238 0.2187
X65 -0.37243 0.31872 -1.169 0.2454
X66 0.36263 0.33034 1.098 0.2750
X67 -0.10120 0.30663 -0.330 0.7421
X68 -0.33790 0.33633 -1.005 0.3175
X69 -0.05326 0.30171 -0.177 0.8602
X70 -0.01047 0.33111 -0.032 0.9748
X71 -0.46896 0.32387 -1.448 0.1508
X72 -0.29867 0.33543 -0.890 0.3754
X73 -0.32556 0.33183 -0.981 0.3289
X74 0.21187 0.31690 0.669 0.5053
X75 0.63659 0.31144 2.044 0.0436 *
X76 0.13838 0.31642 0.437 0.6628
X77 -0.18846 0.29382 -0.641 0.5227
X78 0.06325 0.29180 0.217 0.8289
X79 0.07256 0.30145 0.241 0.8103
X80 0.33483 0.34426 0.973 0.3331
X81 -0.33944 0.35373 -0.960 0.3396
X82 -0.01291 0.32483 -0.040 0.9684
X83 -0.06540 0.27637 -0.237 0.8134
X84 0.11543 0.32813 0.352 0.7257
X85 -0.20415 0.31476 -0.649 0.5181
X86 0.04202 0.33588 0.125 0.9007
X87 -0.33265 0.29159 -1.141 0.2567
X88 -0.49522 0.31251 -1.585 0.1162
X89 -0.39293 0.33358 -1.178 0.2417
X90 -0.34512 0.31892 -1.082 0.2818
X91 0.10540 0.28191 0.374 0.7093
X92 -0.08630 0.30297 -0.285 0.7764
X93 0.02402 0.32907 0.073 0.9420
X94 0.51255 0.32139 1.595 0.1139
X95 -0.19971 0.30634 -0.652 0.5160
X96 -0.09592 0.34585 -0.277 0.7821
X97 -0.18862 0.29266 -0.644 0.5207
X98 0.14997 0.34858 0.430 0.6680
X99 -0.08061 0.30400 -0.265 0.7914
X100 -0.34988 0.31664 -1.105 0.2718
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Residual standard error: 0.9059 on 99 degrees of freedom
Multiple R-squared: 0.4387, Adjusted R-squared: -0.1282
F-statistic: 0.7739 on 100 and 99 DF, p-value: 0.8987But, if we first perform stepwise selection, we end up with 15 out of 28 predictors that have p-values < 0.05.
# Perform model selection using AIC and then summarize the results
selection <- stepAIC(fullModel, trace=0)
summary(selection)
Call:
lm(formula = y ~ X1 + X2 + X3 + X5 + X7 + X13 + X20 + X23 + X30 +
X37 + X42 + X45 + X46 + X47 + X48 + X64 + X65 + X66 + X71 +
X75 + X80 + X81 + X87 + X88 + X89 + X90 + X94 + X100, data = dat)
Residuals:
Min 1Q Median 3Q Max
-2.04660 -0.50885 0.05722 0.49612 1.53704
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 1.0314 0.5045 2.044 0.04244 *
X1 0.4728 0.2185 2.164 0.03187 *
X2 -0.3809 0.2012 -1.893 0.06008 .
X3 0.3954 0.1973 2.004 0.04668 *
X5 -0.2742 0.1861 -1.473 0.14251
X7 -0.4442 0.1945 -2.284 0.02359 *
X13 0.4396 0.1980 2.220 0.02775 *
X20 0.3984 0.1918 2.078 0.03924 *
X23 -0.4137 0.2081 -1.988 0.04836 *
X30 -0.3750 0.1991 -1.884 0.06125 .
X37 0.4006 0.1989 2.015 0.04550 *
X42 -0.3934 0.2021 -1.946 0.05325 .
X45 -0.3197 0.2063 -1.550 0.12296
X46 0.3673 0.1992 1.844 0.06690 .
X47 -0.4240 0.2029 -2.090 0.03811 *
X48 0.5130 0.1937 2.649 0.00884 **
X64 -0.3676 0.2094 -1.755 0.08102 .
X65 -0.2887 0.1975 -1.462 0.14561
X66 0.2769 0.2107 1.315 0.19039
X71 -0.5301 0.2003 -2.646 0.00891 **
X75 0.5020 0.1969 2.550 0.01165 *
X80 0.3722 0.2058 1.809 0.07224 .
X81 -0.3731 0.2176 -1.715 0.08820 .
X87 -0.2684 0.1958 -1.371 0.17225
X88 -0.4524 0.2069 -2.187 0.03011 *
X89 -0.4123 0.2060 -2.002 0.04691 *
X90 -0.3528 0.2067 -1.707 0.08971 .
X94 0.3813 0.2049 1.861 0.06440 .
X100 -0.4058 0.2024 -2.005 0.04653 *
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Residual standard error: 0.76 on 171 degrees of freedom
Multiple R-squared: 0.3177, Adjusted R-squared: 0.2059
F-statistic: 2.843 on 28 and 171 DF, p-value: 1.799e-05One reason why we “discovered” so many “statistically significant” relationships (even when we simulated our response variable completely independent of all 100 predictors), is that we end up with a biased estimate of
8.4.2 Augmented backward elimination
Heinze, Wallisch, and Dunkler (2018) provide a nice overview of methods for selecting explanatory variables, including stepwise approaches (Section 8.4.1), as well as other modeling strategies considered in this Chapter, including full model inference informed by an effective sample size (Section 8.5), AIC-based model weights (Section 8.6), and penalized likelihood methods (Section 8.7). They also highlight an augmented backwards elimination algorithm that adds a “change-in-estimate criterion” to the normal backwards selection algorithm covered in the last section. Variables that, when dropped, result in large changes in other coefficients are retained even if dropping them results in a lower AIC. This strategy is meant to guard against bias incurred by dropping important confounding variables while still allowing one to drop variables that do not aid in predicting the response as long as they do not influence other coefficients in the model.
This method is available in the abe package (Rok Blagus 2017). One can choose to force inclusion of variables that are of particular importance (e.g., treatment variables or known confounders), referred to as “passive” variables (using the include argument). Other variables will be “active” and considered for potential exclusion using significance tests or comparisons using AIC with the added condition that dropping them from the model does not result in a large change to coefficients association with passive variables (where “large” is controlled by a threshold parametervia the argument tau). We demonstrate the approach with the sleep data set, below:
library(abe)
fullmodel <- lm(total_sleep ~ life_span + gestation + log(brain_wt) +
log(body_wt) + predation + exposure + danger, data=mammalsc,
x = TRUE, y = TRUE)
abe(fullmodel, criterion="AIC", verbose = TRUE, exp.beta = FALSE, data=mammalsc)
Model under investigation:
lm(formula = total_sleep ~ life_span + gestation + log(brain_wt) +
log(body_wt) + predation + exposure + danger, data = mammalsc,
x = TRUE, y = TRUE)
Criterion for non-passive variables: life_span : 98.5984 , gestation : 100.1916 , log(brain_wt) : 100.0793 , log(body_wt) : 98.4907 , predation : 102.4047 , exposure : 100.8465 , danger : 109.3066
black list: log(body_wt) : -1.9449, life_span : -1.8371, log(brain_wt) : -0.3562, gestation : -0.244
Investigating change in b or exp(b) due to omitting variable log(body_wt) ; life_span : 0.0075, gestation : 0.0027, log(brain_wt) : 0.0694, predation : 0.0024, exposure : 0.0056, danger : 0.0022
updated black list: life_span : -1.8371, log(brain_wt) : -0.3562, gestation : -0.244
Investigating change in b or exp(b) due to omitting variable life_span ; gestation : 0.0133, log(brain_wt) : 0.0615, log(body_wt) : 0.0273, predation : 0.0129, exposure : 0.0028, danger : 0.0026
updated black list: log(brain_wt) : -0.3562, gestation : -0.244
Investigating change in b or exp(b) due to omitting variable log(brain_wt) ; life_span : 0.0837, gestation : 0.0288, log(body_wt) : 0.3424, predation : 0.0075, exposure : 0.0257, danger : 0.0103
updated black list: gestation : -0.244
Investigating change in b or exp(b) due to omitting variable gestation ; life_span : 0.0422, log(brain_wt) : 0.0669, log(body_wt) : 0.0313, predation : 0.0774, exposure : 0.027, danger : 0.0838
Final model:
lm(formula = total_sleep ~ life_span + gestation + log(brain_wt) +
log(body_wt) + predation + exposure + danger, data = mammalsc,
x = TRUE, y = TRUE)
Call:
lm(formula = total_sleep ~ life_span + gestation + log(brain_wt) +
log(body_wt) + predation + exposure + danger, data = mammalsc,
x = TRUE, y = TRUE)
Coefficients:
(Intercept) life_span gestation log(brain_wt) log(body_wt) predation exposure danger
16.91558 0.01393 -0.00767 -0.85003 0.11271 2.00018 0.98176 -4.27035 In the output, above, variables that, when dropped, lead to increases in AIC are “blacklisted” and therefore not considered for potential exclusion (the same would be true for variables listed as passive using the include argument). The algorithm then looks to see whether dropping any of the variables that improve AIC result in significant changes to other non-passive variables. In this case, we see that we do not eliminate any of the explanatory variables as doing so results in large changes in one or more of the passive variables. We could relax the change-in-estimate criterion by specifying a larger value of tau than the default (0.05). If we use a value of 0.1, then we end up dropping log(body_wt) and life_span:
abe(fullmodel, criterion="AIC", verbose = TRUE, exp.beta = FALSE, tau= 0.1, data=mammalsc)
Model under investigation:
lm(formula = total_sleep ~ life_span + gestation + log(brain_wt) +
log(body_wt) + predation + exposure + danger, data = mammalsc,
x = TRUE, y = TRUE)
Criterion for non-passive variables: life_span : 98.5984 , gestation : 100.1916 , log(brain_wt) : 100.0793 , log(body_wt) : 98.4907 , predation : 102.4047 , exposure : 100.8465 , danger : 109.3066
black list: log(body_wt) : -1.9449, life_span : -1.8371, log(brain_wt) : -0.3562, gestation : -0.244
Investigating change in b or exp(b) due to omitting variable log(body_wt) ; life_span : 0.0075, gestation : 0.0027, log(brain_wt) : 0.0694, predation : 0.0024, exposure : 0.0056, danger : 0.0022
Model under investigation:
lm(formula = total_sleep ~ life_span + gestation + log(brain_wt) +
predation + exposure + danger, data = mammalsc, x = TRUE,
y = TRUE)
Criterion for non-passive variables: life_span : 96.6211 , gestation : 98.2091 , log(brain_wt) : 100.5057 , predation : 100.4301 , exposure : 99.0145 , danger : 107.3227
black list: life_span : -1.8695, gestation : -0.2816
Investigating change in b or exp(b) due to omitting variable life_span ; gestation : 0.0113, log(brain_wt) : 0.0328, predation : 0.011, exposure : 0.0044, danger : 0.0016
Model under investigation:
lm(formula = total_sleep ~ gestation + log(brain_wt) + predation +
exposure + danger, data = mammalsc, x = TRUE, y = TRUE)
Criterion for non-passive variables: gestation : 96.218 , log(brain_wt) : 98.9825 , predation : 98.4525 , exposure : 97.0796 , danger : 105.4001
black list: gestation : -0.4031
Investigating change in b or exp(b) due to omitting variable gestation ; log(brain_wt) : 0.1182, predation : 0.0846, exposure : 0.0255, danger : 0.084
Final model:
lm(formula = total_sleep ~ gestation + log(brain_wt) + predation +
exposure + danger, data = mammalsc, x = TRUE, y = TRUE)
Call:
lm(formula = total_sleep ~ gestation + log(brain_wt) + predation +
exposure + danger, data = mammalsc, x = TRUE, y = TRUE)
Coefficients:
(Intercept) gestation log(brain_wt) predation exposure danger
16.759945 -0.007153 -0.658072 1.956738 0.985174 -4.257452 8.5 Degrees of freedom (df) spending: One model to rule them all
Given the potential for overfitting and issues with multiple testing and inference when using model selection algorithms, it can be advantageous at times to just fit a single model and use it for inference (Babyak 2004; Whittingham et al. 2006; Giudice, Fieberg, and Lenarz 2012; Harrell Jr 2015; Fieberg and Johnson 2015). In fact, this is the approach I suggest for those that feel they want to do a bit of everything (describe, predict, and infer). We can use the fitted coefficients and their signs to describe associations between our explanatory variables and the response variable. Because the model is pre-specified, confidence intervals and p-values would have their correct interpretation as long as the assumptions are not violated. In other words, rather than use model selection to determine whether associations are “significant” or not, we can judge significance using estimates of effect size (i.e., regression coefficients) and their uncertainty. If our goal is prediction, then we may lose out on some precision gains that could result from dropping unimportant predictors. However, this gain is usually minimal and probably not worth the cost (e.g., biased regression coefficients, p-values that are too small, etc).
Yet, this approach is not without its challenges, particularly when there are a large number of potential explanatory variables to choose from. Fitting a single model with too many predictors can be problematic for reasons discussed previously (e.g., collinearity, potential for overfitting). Thus, an important first step is usually to whittle down the number of explanatory variables to consider. During this step, one should also consider whether some variables are likely to have a non-linear relationship with the response variable (Chapter 4). The number of explanatory variables and the degree of flexibility allowed for modeling associations should ideally be informed by the effective sample size available for estimating model parameters, which will depend on the type of response variable (Table 8.2 recreated from Harrell Jr (2015)). For continuous response variables with independent observations, the effective sample size is simply the number of observations. For binary data (0’s and 1’s), the minimum of the 0’s and 1’s is a better measure of effective sample size; we have no ability to discover important associations when all responses are either 0 or 1. General guidelines have been proposed in the literature based on theory and simulation studies that one should limit the number of model degrees of freedom (think “number of parameters”) to
| Response | Effective Degrees of Freedom |
|---|---|
| Continuous | |
| Binary | min( |
| Ordinal ( |
|
| Failure (survival time) | Number of failures |
When determining which variables to include, it is important that we do not consider the strength of the relationship between the explanatory variables and the response variable since this data-driven approach will potentially lead to overfitting and negate the benefits of avoiding model selection. Instead, we should consider subject matter knowledge (i.e., include variables likely to be important based on prior work), cost/feasibility of data collection, relevance to your research questions and potential to be a confounding variable. In addition, we might rule out variables that have a lot of missing data, that are highly correlated with one or more other variables, or that vary little. In some cases, we might consider using principle components or other indexing methods to reduce the number of predictors (Section 6.9 and Section 6.10). Lastly, Dormann et al. (2013) suggest:
In any regression-style model, the results will be most informative if predictors that are directly relevant to the response are used, i.e. proximal predictors are strongly preferable over distal ones (Austin 1980, 2002). This general concept leads to careful consideration of candidate predictor sets in the light of ecological knowledge, rather than amassing whatever data can be found and challenging the model to make sense of it.” and…“As a general rule of thumb, a good strategy is to select variables that a) are ecologically relevant, b) are feasible to collect data on and c) are closer to the mechanism (in the sequence resource-direct-indirect-proxy variables: Harrell 2001, Austin 2002). Then, if the statistical method suggests deleting an ecologically reasonable or important variable, prominence should be given to ecology.
To summarize this approach to modeling:
- Limit model df (number of parameters) to
- Fit a “full model” without further simplification.
- Determine important associations between explanatory and response variables using measures of effect size and their uncertainty.
In other words, determine how many ‘degrees of freedom’ you can spend, spend them, and then don’t look back. If you feel you must look at other predictors or models, do this as part of a secondary “exploratory analysis” or “sensitivity analysis”, and treat any discoveries during this secondary phase with caution. If you find yourself modeling with fewer events per variable than recommended (e.g., EPV < 10-20), then you should consider evaluating model stability using a bootstrap (see Section 8.8.2) or consider using some form of model-averaging (Section 8.6) or shrinkage estimator like the LASSO (Section 8.7) (Heinze, Wallisch, and Dunkler 2018).
8.6 AIC and model-averaging
We have just considered two modeling strategies at the opposite ends of the spectrum, stepwise-selection algorithms using AIC and inference based on a single model. One of the main downsides that we highlighted with using a single model was that prediction errors may be larger than necessary due to including predictors that just add noise. We also remarked that there is usually little cost to including predictors that explain little variation in the response. The Akaike information criterion (AIC) (Akaike 1974) is often used to choose among competing models, not just nested models when applying a stepwise-selection algorithm. The AIC was derived to measure the quality of future predictions, and thus, it is not surprising that AIC tends to select bigger models than a selection algorithm that uses null hypothesis tests. For example, if we use AIC to distinguish between two nested models that differ by 1 parameter, this is equivalent to choosing the larger model whenever a likelihood ratio test has a p-value < 0.157 (Anderson and Burnham 2004)3.
If we are truly interested in prediction, there are alternatives we might want consider, including model-averaging and various “regularization techniques” that effectively “shrink” estimates associated with weakly-informative parameters towards 0 (Dahlgren 2010; Hooten and Hobbs 2015; Lever, Krzywinski, and Altman 2016). Alternatively, there are a number of non-parametric approaches (e.g., boosted regression trees, random forests, etc) that tend to perform extremely well when it comes to prediction (Cutler et al. 2007; Elith, Leathwick, and Hastie 2008; Lucas 2020). The main downside of these latter approaches is that they can lack clearly interpretable parameters that describe relationships between explanatory and response variables.
Here, we will briefly consider model-averaging using AIC-based model weights. This approach become incredibly popular among wildlife ecologists in the early 2000’s following the publication of Anderson and Burnham (2004) (and other papers and an earlier edition that preceded it). In fact, there was a time when it was really difficult to get anything published in the Journal of Wildlife Management if you choose to use a different strategy for analyzing your data. During the past 10 years or so, there have been many critiques of how AIC is often used by wildlife ecologists (Arnold 2010; Murtaugh 2014; Cade 2015; Brewer, Butler, and Cooksley 2016), and AIC-based model averaging is no longer so prominent.
Rather than choose a best model, we can choose to average predictions among multiple “good” models. Buckland, Burnham, and Augustin (1997) and Anderson and Burnham (2004) suggested weighting models using AIC. Specifically, they outlined the following approach:
- Write down
- Fit these models and calculate
- Compute model weights, using the
where
- Calculate a model-averaged parameter using the weights and parameter estimates from the
- Calculate a standard error (SE) that accounts for model and sampling uncertainty:
Typically, one would also form 95% CIs using
We can implement this approach using the MuMin package (Barton 2020) as demonstrated below with the sleep data set.
library(MuMIn)We begin by fitting all possible models including one or more candidate predictors from our full model fit to the sleep data using the dredge function.
options(na.action = "na.fail")
fullmodel<-lm(total_sleep ~ life_span + gestation + log(brain_wt) +
log(body_wt) + predation + exposure + danger, data=mammalsc)
allsubsets <- dredge(fullmodel)Fixed term is "(Intercept)"allsubsetsGlobal model call: lm(formula = total_sleep ~ life_span + gestation + log(brain_wt) +
log(body_wt) + predation + exposure + danger, data = mammalsc)
---
Model selection table
(Int) dng exp gst lif_spn log(bdy_wt) log(brn_wt) prd df logLik AICc delta weight
98 16.40 -3.628 -0.6763 1.99200 5 -103.722 219.1 0.00 0.102
100 16.59 -4.543 0.90660 -0.8800 2.23200 6 -102.704 219.8 0.70 0.072
70 16.09 -3.742 -0.012360 2.11000 5 -104.384 220.4 1.33 0.053
34 17.44 -1.575 -0.9194 4 -105.692 220.5 1.35 0.052
102 16.54 -3.309 -0.006265 -0.4665 1.73300 6 -103.135 220.7 1.56 0.047
38 17.44 -1.504 -0.008654 -0.5858 5 -104.598 220.9 1.75 0.042
82 14.78 -3.837 -0.495600 2.23200 5 -104.658 221.0 1.87 0.040
104 16.76 -4.257 0.98520 -0.007153 -0.6581 1.95700 7 -101.906 221.1 2.00 0.038
86 15.60 -3.384 -0.008064 -0.285800 1.83600 6 -103.642 221.7 2.57 0.028
114 16.80 -3.663 0.141400 -0.8359 2.00400 6 -103.679 221.8 2.65 0.027
106 16.39 -3.629 0.001225 -0.6836 1.99700 6 -103.721 221.8 2.73 0.026
40 17.72 -2.090 0.80650 -0.009634 -0.7553 6 -103.822 222.0 2.93 0.024
36 17.67 -2.061 0.65970 -1.0890 5 -105.193 222.1 2.94 0.023
6 17.10 -1.558 -0.017460 4 -106.513 222.1 3.00 0.023
22 16.28 -1.459 -0.010860 -0.380000 5 -105.249 222.2 3.06 0.022
84 14.51 -4.615 0.77180 -0.638400 2.45400 6 -103.959 222.3 3.21 0.021
72 16.10 -4.273 0.46450 -0.013960 2.28800 6 -104.087 222.6 3.46 0.018
88 15.40 -4.238 0.90140 -0.009041 -0.427100 2.04700 7 -102.650 222.6 3.48 0.018
116 16.73 -4.546 0.89750 0.052610 -0.9373 2.23400 7 -102.698 222.7 3.58 0.017
108 16.55 -4.548 0.90900 0.003185 -0.8995 2.24600 7 -102.700 222.7 3.58 0.017
78 16.37 -3.675 -0.010680 -0.017440 1.99500 6 -104.225 222.9 3.74 0.016
42 17.53 -1.602 -0.008602 -0.8641 5 -105.663 223.0 3.88 0.015
50 17.72 -1.592 0.101000 -1.0340 5 -105.672 223.0 3.90 0.015
18 15.29 -1.514 -0.715000 4 -107.059 223.2 4.09 0.013
90 15.26 -3.768 -0.018450 -0.419600 2.10600 6 -104.486 223.4 4.26 0.012
118 17.00 -3.345 -0.006350 0.164100 -0.6489 1.74300 7 -103.077 223.4 4.34 0.012
110 16.44 -3.303 -0.006559 0.008853 -0.5092 1.75700 7 -103.103 223.5 4.39 0.011
54 17.84 -1.526 -0.008738 0.139500 -0.7415 6 -104.559 223.5 4.41 0.011
46 17.41 -1.493 -0.008772 0.003183 -0.6018 6 -104.594 223.6 4.48 0.011
24 16.18 -1.955 0.70660 -0.011880 -0.499300 6 -104.678 223.8 4.65 0.010
14 17.48 -1.646 -0.014130 -0.029600 5 -106.078 223.8 4.71 0.010
74 16.20 -4.615 -0.051960 2.66400 5 -106.147 224.0 4.85 0.009
112 16.63 -4.263 0.99890 -0.007569 0.012180 -0.7196 1.99300 8 -101.841 224.0 4.94 0.009
120 16.95 -4.262 0.97320 -0.007178 0.070800 -0.7344 1.95800 8 -101.895 224.2 5.04 0.008
94 15.71 -3.381 -0.007776 -0.005091 -0.272300 1.81500 7 -103.629 224.6 5.44 0.007
8 17.14 -1.676 0.15800 -0.018150 5 -106.480 224.6 5.52 0.006
122 16.78 -3.669 0.003473 0.151600 -0.8681 2.01900 7 -103.674 224.6 5.53 0.006
44 17.74 -2.085 0.65740 -0.008068 -1.0360 6 -105.167 224.7 5.62 0.006
30 16.52 -1.506 -0.010070 -0.012620 -0.344000 6 -105.178 224.8 5.65 0.006
52 17.76 -2.062 0.65380 0.032970 -1.1250 6 -105.190 224.8 5.67 0.006
92 15.03 -4.560 0.79210 -0.020270 -0.558700 2.32100 7 -103.745 224.8 5.67 0.006
26 16.09 -1.623 -0.032410 -0.559800 5 -106.560 224.8 5.68 0.006
48 17.67 -2.075 0.81110 -0.009833 0.005268 -0.7827 7 -103.810 224.9 5.80 0.006
56 17.89 -2.092 0.79610 -0.009657 0.060700 -0.8209 7 -103.814 224.9 5.81 0.006
66 14.89 -5.547 3.61500 4 -107.948 225.0 5.87 0.005
80 16.45 -4.261 0.52590 -0.012110 -0.021400 2.17100 7 -103.848 225.0 5.88 0.005
20 15.16 -1.847 0.47070 -0.815400 5 -106.821 225.3 6.20 0.005
96 15.51 -4.236 0.90250 -0.008729 -0.005529 -0.412600 2.02400 8 -102.635 225.6 6.52 0.004
58 17.74 -1.612 -0.007476 0.079680 -0.9621 6 -105.651 225.7 6.59 0.004
124 16.72 -4.555 0.89850 0.004121 0.064630 -0.9756 2.25200 8 -102.691 225.7 6.64 0.004
101 17.35 -0.010950 -0.6853 -1.14400 5 -107.304 226.3 7.16 0.003
16 17.57 -1.858 0.27410 -0.015040 -0.032230 6 -105.981 226.4 7.25 0.003
62 17.83 -1.509 -0.008956 0.005644 0.156600 -0.7889 7 -104.547 226.4 7.28 0.003
126 16.96 -3.346 -0.006769 0.012080 0.201200 -0.7485 1.77700 8 -103.019 226.4 7.29 0.003
32 16.44 -2.011 0.71440 -0.011030 -0.013650 -0.461600 7 -104.592 226.5 7.37 0.003
76 16.22 -4.797 0.13400 -0.054150 2.73100 6 -106.122 226.6 7.53 0.002
97 17.24 -1.1200 -1.17900 4 -108.851 226.8 7.67 0.002
28 15.99 -2.009 0.53310 -0.034600 -0.663000 6 -106.250 226.9 7.79 0.002
128 16.92 -4.270 0.98180 -0.007670 0.013930 0.112700 -0.8500 2.00000 9 -101.813 227.3 8.14 0.002
68 14.97 -5.108 -0.26650 3.40100 5 -107.847 227.4 8.25 0.002
85 15.90 -0.012910 -0.472600 -1.08200 5 -107.858 227.4 8.27 0.002
60 17.77 -2.085 0.65570 -0.007926 0.010150 -1.0480 7 -105.166 227.6 8.52 0.001
10 17.79 -2.032 -0.086870 4 -109.414 227.9 8.80 0.001
64 17.88 -2.074 0.79840 -0.009911 0.006495 0.080140 -0.8756 8 -103.798 228.0 8.85 0.001
69 16.81 -0.021460 -1.14500 4 -109.601 228.3 9.17 0.001
109 17.23 -0.011300 0.010940 -0.7378 -1.10900 6 -107.263 228.9 9.82 0.001
103 17.32 -0.08041 -0.010770 -0.6646 -1.09500 6 -107.295 229.0 9.88 0.001
117 17.42 -0.010970 0.022330 -0.7105 -1.14700 6 -107.303 229.0 9.90 0.001
99 17.11 -0.31410 -1.0110 -0.98480 5 -108.723 229.1 10.00 0.001
81 14.52 -0.881400 -1.07300 4 -110.106 229.3 10.18 0.001
113 17.12 -0.044840 -1.0680 -1.17300 5 -108.848 229.4 10.25 0.001
105 17.28 -0.002831 -1.1030 -1.18800 5 -108.849 229.4 10.25 0.001
7 15.40 -1.23600 -0.014170 4 -110.220 229.5 10.41 0.001
71 16.66 -0.61020 -0.017660 -0.77220 5 -109.035 229.7 10.63 0.001
35 15.46 -1.18500 -0.7263 4 -110.463 230.0 10.90 0.000
87 15.92 -0.14950 -0.012540 -0.441300 -0.99540 6 -107.829 230.1 10.95 0.000
93 16.03 -0.012520 -0.006672 -0.454700 -1.10600 6 -107.840 230.1 10.97 0.000
77 17.25 -0.018410 -0.028810 -1.23800 5 -109.256 230.2 11.07 0.000
12 17.66 -1.746 -0.33080 -0.079280 5 -109.275 230.2 11.11 0.000
39 15.48 -1.07100 -0.009029 -0.4090 5 -109.535 230.7 11.63 0.000
19 13.87 -1.17600 -0.568500 4 -111.003 231.1 11.98 0.000
23 14.69 -1.05600 -0.010230 -0.283800 5 -109.717 231.1 11.99 0.000
89 15.32 -0.030120 -0.746100 -1.18200 5 -109.748 231.2 12.05 0.000
83 14.71 -0.47020 -0.746700 -0.79970 5 -109.834 231.3 12.22 0.000
111 17.20 -0.06962 -0.011140 0.010670 -0.7185 -1.06800 7 -107.257 231.8 12.70 0.000
125 17.38 -0.011380 0.011900 0.058780 -0.8085 -1.11400 7 -107.257 231.8 12.70 0.000
107 17.14 -0.31490 -0.003154 -0.9916 -0.99420 6 -108.720 231.8 12.73 0.000
115 17.11 -0.31450 0.002015 -1.0130 -0.98480 6 -108.723 231.8 12.74 0.000
119 17.41 -0.08668 -0.010780 0.034100 -0.7014 -1.09600 7 -107.293 231.9 12.77 0.000
79 17.02 -0.54130 -0.015670 -0.022860 -0.88800 6 -108.820 232.0 12.93 0.000
121 17.13 -0.003633 -0.055190 -1.0340 -1.18300 6 -108.844 232.1 12.98 0.000
15 15.42 -1.23900 -0.013850 -0.002928 5 -110.217 232.1 12.99 0.000
43 15.37 -1.14800 0.012690 -0.8154 5 -110.412 232.5 13.38 0.000
51 15.42 -1.18200 -0.014570 -0.7104 5 -110.463 232.6 13.48 0.000
95 16.05 -0.14760 -0.012170 -0.006535 -0.424200 -1.02000 7 -107.813 232.9 13.81 0.000
47 15.31 -0.98250 -0.010040 0.026210 -0.5573 6 -109.316 233.0 13.92 0.000
91 15.43 -0.42200 -0.027840 -0.635500 -0.92850 6 -109.527 233.5 14.34 0.000
55 15.51 -1.07300 -0.009033 0.010750 -0.4205 6 -109.535 233.5 14.36 0.000
27 14.07 -1.19800 -0.009217 -0.522100 5 -110.968 233.6 14.49 0.000
21 12.73 -0.012670 -0.546800 4 -112.317 233.7 14.61 0.000
31 14.51 -1.01900 -0.010990 0.011680 -0.321300 6 -109.664 233.7 14.62 0.000
37 14.13 -0.012120 -0.6856 4 -112.389 233.9 14.75 0.000
11 15.62 -1.71400 -0.048100 4 -112.564 234.2 15.10 0.000
33 13.90 -1.1690 3 -113.885 234.4 15.29 0.000
3 15.16 -1.91600 3 -113.925 234.5 15.37 0.000
4 16.18 -1.024 -1.18100 4 -112.719 234.5 15.41 0.000
123 17.12 -0.31350 -0.003261 -0.007425 -0.9829 -0.99440 7 -108.720 234.7 15.62 0.000
45 14.01 -0.013620 0.051600 -0.9327 5 -111.566 234.8 15.69 0.000
17 11.40 -0.947400 3 -114.089 234.8 15.70 0.000
127 17.38 -0.08129 -0.011200 0.011750 0.069360 -0.7987 -1.06600 8 -107.249 234.9 15.75 0.000
5 13.58 -0.022640 3 -114.214 235.1 15.95 0.000
59 15.44 -1.15200 0.013020 0.023760 -0.8435 6 -110.411 235.2 16.11 0.000
75 16.81 -1.24800 -0.067100 -0.65210 5 -111.881 235.4 16.32 0.000
29 12.42 -0.014480 0.031160 -0.622500 5 -111.966 235.6 16.49 0.000
2 16.16 -2.051 3 -114.628 235.9 16.78 0.000
63 15.56 -0.99680 -0.010140 0.027660 0.095260 -0.6678 7 -109.303 235.9 16.79 0.000
41 13.79 0.038270 -1.3960 4 -113.455 236.0 16.88 0.000
53 13.32 -0.011810 -0.330300 -0.3135 5 -112.228 236.1 17.01 0.000
49 12.89 -0.411100 -0.6900 4 -113.650 236.4 17.27 0.000
67 15.29 -1.86500 -0.08522 4 -113.911 236.9 17.79 0.000
25 11.30 0.006779 -0.976300 4 -114.072 237.2 18.12 0.000
61 13.68 -0.013410 0.048970 -0.137800 -0.7649 6 -111.539 237.5 18.37 0.000
13 13.55 -0.023240 0.005887 4 -114.202 237.5 18.37 0.000
73 17.38 -0.108000 -1.57300 4 -114.356 237.8 18.68 0.000
57 13.10 0.033200 -0.288100 -1.0310 5 -113.345 238.4 19.25 0.000
65 14.53 -1.31700 3 -120.415 247.5 28.35 0.000
9 12.37 -0.088910 3 -120.850 248.3 29.22 0.000
1 10.64 2 -124.171 252.6 33.54 0.000
Models ranked by AICc(x) We see a list of all models, sorted by AIC from “best” to “worst.” Interestingly, we find that the top model differs from the one chosen using forwards- or backwards-stepwise selection due to using the small sample size correction for AIC. In this case, the model containing log(brain_wt), predation, and danger has the smallest AIC by 0.7 units and gets the largest weight of 0.102. Yet, several other models have similar AICc values and weights ranging from 0.001 to 0.072. Rather than average across all models, some of which have weights very close to 0, we could choose to average across all models with
modaverage <- model.avg(allsubsets, subset = delta < 4)
summary(modaverage)
Call:
model.avg(object = allsubsets, subset = delta < 4)
Component model call:
lm(formula = total_sleep ~ <23 unique rhs>, data = mammalsc)
Component models:
df logLik AICc delta weight
167 5 -103.72 219.11 0.00 0.14
1267 6 -102.70 219.81 0.70 0.10
137 5 -104.38 220.44 1.33 0.07
16 4 -105.69 220.46 1.35 0.07
1367 6 -103.14 220.67 1.56 0.06
136 5 -104.60 220.86 1.75 0.06
157 5 -104.66 220.98 1.87 0.05
12367 7 -101.91 221.11 2.00 0.05
1357 6 -103.64 221.68 2.57 0.04
1567 6 -103.68 221.76 2.65 0.04
1467 6 -103.72 221.84 2.73 0.03
1236 6 -103.82 222.04 2.93 0.03
126 5 -105.19 222.05 2.94 0.03
13 4 -106.51 222.11 3.00 0.03
135 5 -105.25 222.17 3.06 0.03
1257 6 -103.96 222.32 3.21 0.03
1237 6 -104.09 222.57 3.46 0.02
12357 7 -102.65 222.59 3.48 0.02
12567 7 -102.70 222.69 3.58 0.02
12467 7 -102.70 222.69 3.58 0.02
1347 6 -104.23 222.85 3.74 0.02
146 5 -105.66 222.99 3.88 0.02
156 5 -105.67 223.01 3.90 0.02
Term codes:
danger exposure gestation life_span log(body_wt) log(brain_wt) predation
1 2 3 4 5 6 7
Model-averaged coefficients:
(full average)
Estimate Std. Error Adjusted SE z value Pr(>|z|)
(Intercept) 16.5255571 1.4629993 1.4981005 11.031 <2e-16 ***
danger -3.2748330 1.5135224 1.5381705 2.129 0.0333 *
log(brain_wt) -0.5227838 0.4728736 0.4785835 1.092 0.2747
predation 1.4758462 1.2955748 1.3161523 1.121 0.2621
exposure 0.2756132 0.5579838 0.5671662 0.486 0.6270
gestation -0.0043163 0.0064913 0.0065641 0.658 0.5108
log(body_wt) -0.0674230 0.2502241 0.2543647 0.265 0.7910
life_span -0.0004167 0.0115914 0.0119608 0.035 0.9722
(conditional average)
Estimate Std. Error Adjusted SE z value Pr(>|z|)
(Intercept) 16.525557 1.462999 1.498101 11.031 <2e-16 ***
danger -3.274833 1.513522 1.538171 2.129 0.0333 *
log(brain_wt) -0.763824 0.377620 0.387988 1.969 0.0490 *
predation 2.066175 1.063102 1.097919 1.882 0.0598 .
exposure 0.841235 0.688824 0.711346 1.183 0.2370
gestation -0.009928 0.006419 0.006587 1.507 0.1318
log(body_wt) -0.271224 0.443391 0.452769 0.599 0.5492
life_span -0.004280 0.036925 0.038116 0.112 0.9106
---
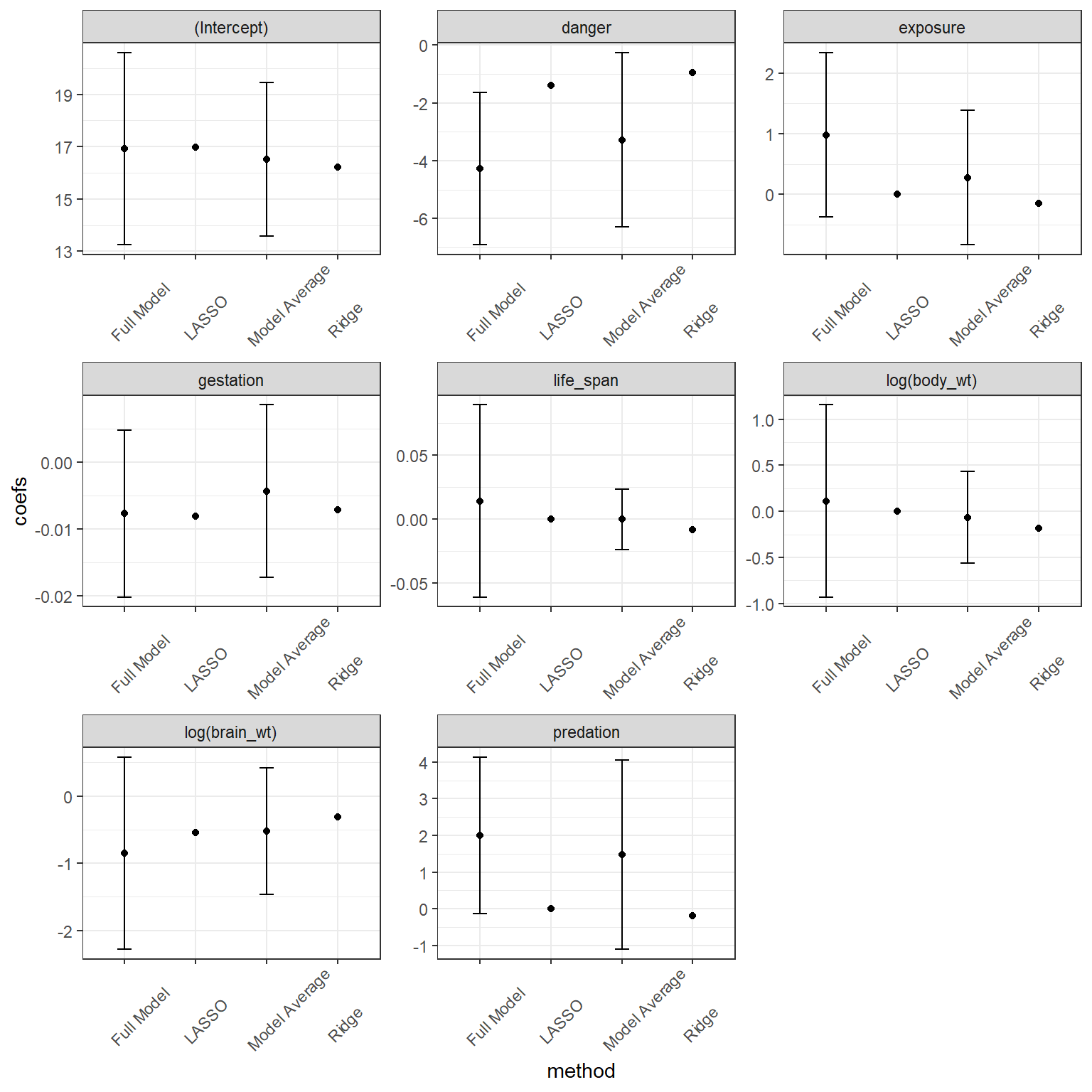
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1We see a list of the models that we average over and also see two sets of averaged coefficients. The set of coefficients listed under (full average) uses 0’s for coefficients when averaging over models that do not contain the focal predictor. By contrast, the set of coefficients under (conditional average) only averages coefficients in the models where the predictor is included. The former approach has a stronger theoretical foundation and results in a similar effect as various penalization strategies (see Section 8.7); namely, this approach to model averaging will shrink parameters associated with weak parameters towards 0 (Lukacs, Burnham, and Anderson 2010). We can see this effect if we plot the model-averaged coefficients against the coefficients from the full model. This “shrinkage” effect turns out to be a useful characteristic that often improves mean-squared prediction error, MSE:
# pull off average coefficients and their SEs
avecoef <- (summary(modaverage))$coefmat.full
#reorder terms so they appear the same as in fullmodelsum, below
avecoef <- avecoef[c(1,8,6,3,7,4,5,2),]
fullmodelsum <- broom::tidy(fullmodel)
combinedcoef <- data.frame(coefs = c(avecoef[,1], fullmodelsum$estimate),
se = c(avecoef[,3], fullmodelsum$std.error),
term = as.factor(rep(fullmodelsum$term, 2)),
method = rep(c("Model Average", "Full Model"), each =8)) %>%
mutate(upci= coefs + 1.96*se,
loci = coefs - 1.96*se)ggplot(combinedcoef, aes(method, coefs)) + geom_point()+
geom_errorbar(aes(ymin = loci, ymax = upci, width = 0.2)) +
facet_wrap(~term, scales="free") +theme_bw()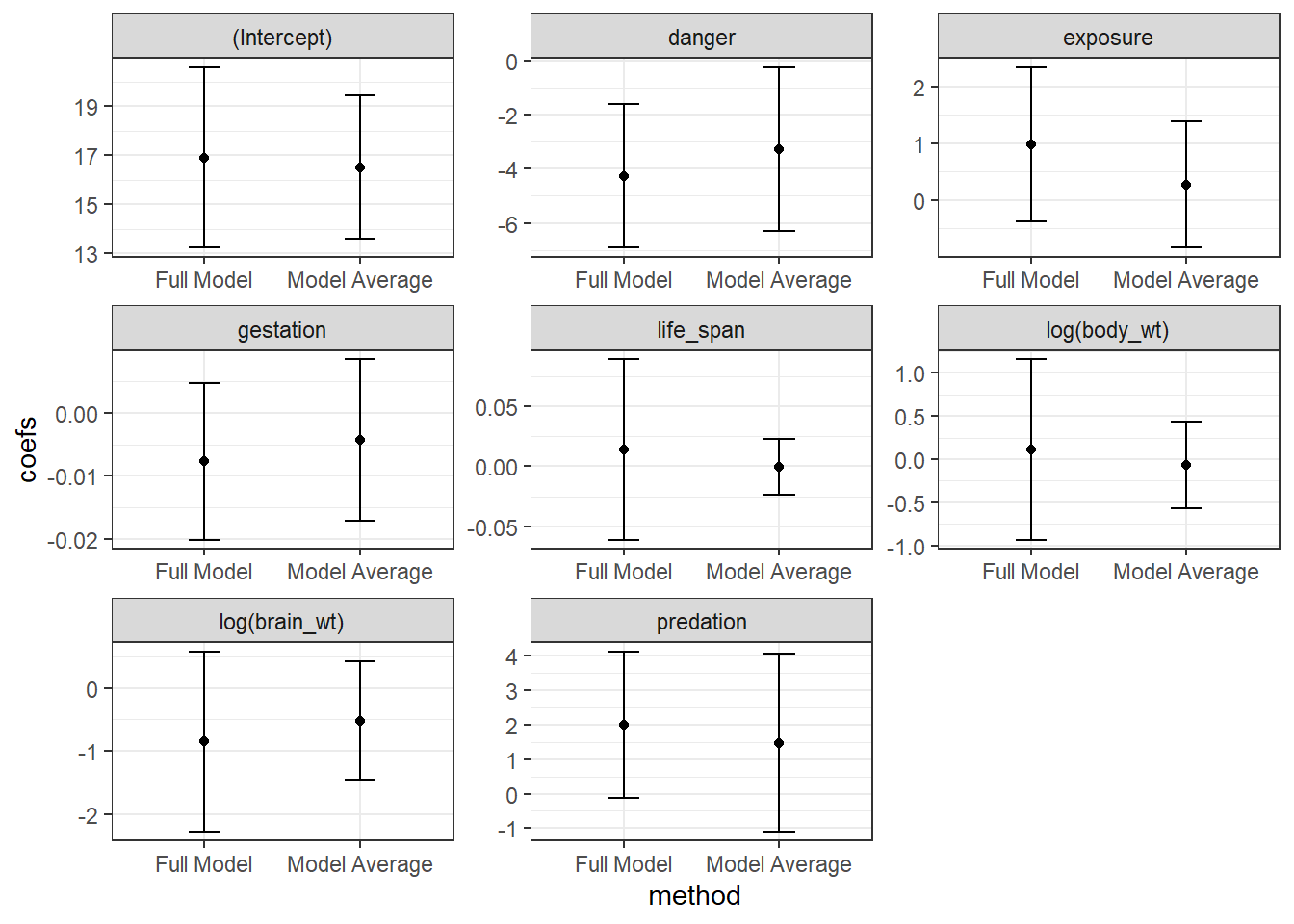
8.7 Regularization using penalization
As mentioned in the previous section, if your goal is prediction, it may be useful to shrink certain coefficients towards 0. One way to accomplish this goal is to add a penalty,
Typically,
Why do shrinkage estimators improve predictive performance? Essentially, shrinkage can be viewed as a method for reducing model complexity; shrinkage reduces variability in the estimates of
Below, we will estimate parameters using the LASSO and Ridge regression applied to the sleep data set. We will make use of the glmnet package (Friedman, Hastie, and Tibshirani 2010), which works with matrices rather than dataframes. Therefore, we will first need to create our logged predictors of body and brain weight. We can specify
library(glmnet)
mammalsc <- mammalsc %>% mutate(logbrain_wt = log(brain_wt),
logbody_wt = log(body_wt))
x = as.matrix(mammalsc[, c("life_span", "gestation", "logbrain_wt",
"logbody_wt", "predation", "exposure", "danger")])
fitlasso <- glmnet(x = x,
y= mammalsc$total_sleep, alpha=1)By default, glmnet will estimate parameters across a range of plot function with argument xvar = "lambda" (Figure 8.3).
plot(fitlasso, xvar = "lambda", label = TRUE)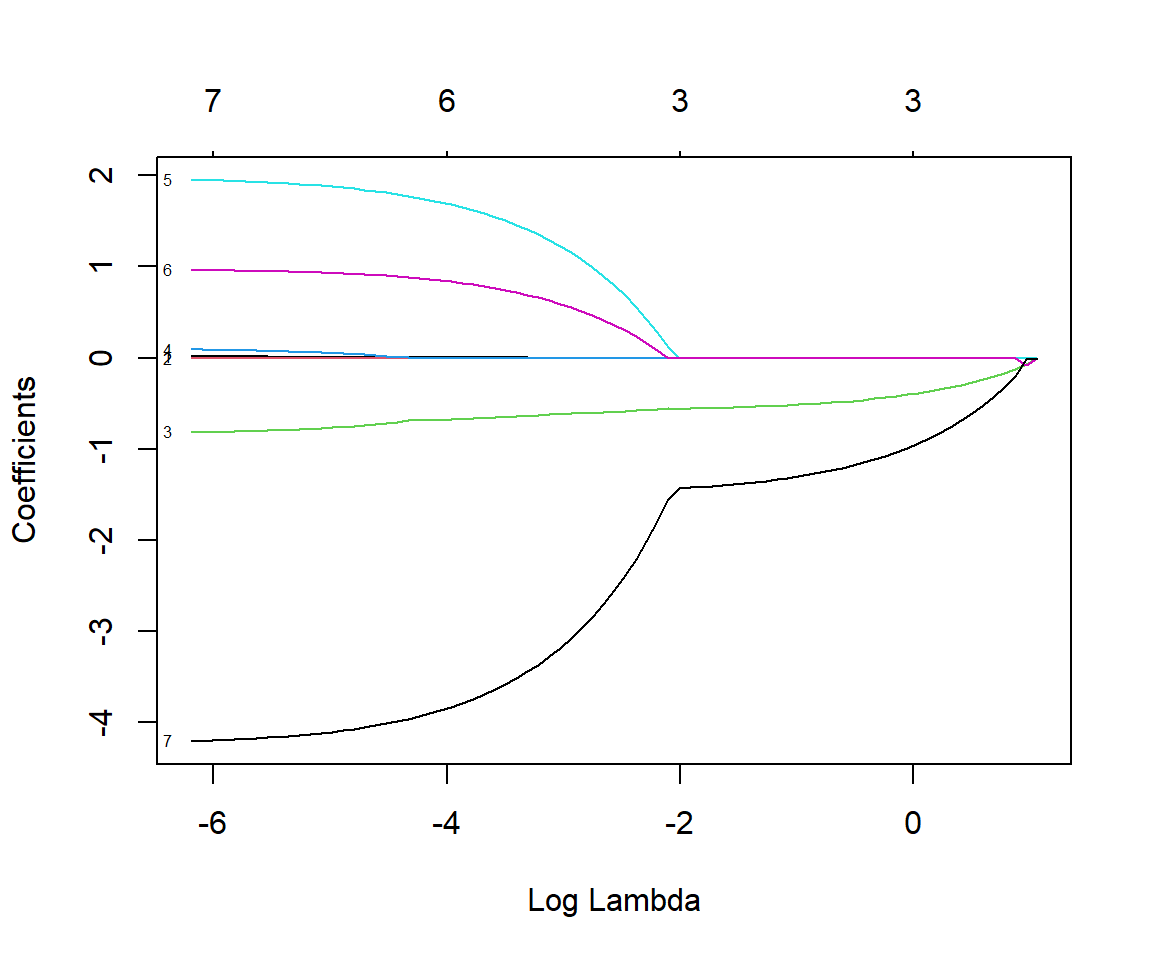
At this point, we have a number of different models, each with a different set of coefficient estimates determined by the unique set of print function.
print(fitlasso)
Call: glmnet(x = x, y = mammalsc$total_sleep, alpha = 1)
Df %Dev Lambda
1 0 0.00 2.89600
2 4 8.32 2.63800
3 4 17.42 2.40400
4 3 24.79 2.19000
5 3 30.87 1.99600
6 3 35.92 1.81900
7 3 40.12 1.65700
8 3 43.60 1.51000
9 3 46.49 1.37600
10 3 48.89 1.25300
11 3 50.88 1.14200
12 3 52.54 1.04100
13 3 53.91 0.94820
14 3 55.05 0.86390
15 3 56.00 0.78720
16 3 56.78 0.71730
17 3 57.44 0.65350
18 3 57.98 0.59550
19 3 58.43 0.54260
20 3 58.80 0.49440
21 3 59.11 0.45050
22 3 59.37 0.41040
23 3 59.58 0.37400
24 3 59.76 0.34080
25 3 59.91 0.31050
26 3 60.03 0.28290
27 3 60.13 0.25780
28 3 60.21 0.23490
29 3 60.28 0.21400
30 3 60.34 0.19500
31 3 60.39 0.17770
32 3 60.43 0.16190
33 3 60.46 0.14750
34 3 60.49 0.13440
35 4 60.85 0.12250
36 5 61.60 0.11160
37 5 62.24 0.10170
38 5 62.76 0.09264
39 5 63.20 0.08441
40 5 63.57 0.07691
41 5 63.87 0.07008
42 5 64.12 0.06385
43 5 64.33 0.05818
44 5 64.51 0.05301
45 5 64.65 0.04830
46 5 64.77 0.04401
47 6 64.89 0.04010
48 6 64.99 0.03654
49 6 65.07 0.03329
50 6 65.13 0.03033
51 6 65.19 0.02764
52 6 65.24 0.02518
53 6 65.28 0.02295
54 6 65.31 0.02091
55 6 65.34 0.01905
56 6 65.36 0.01736
57 6 65.38 0.01582
58 6 65.39 0.01441
59 7 65.41 0.01313
60 7 65.43 0.01196
61 7 65.44 0.01090
62 7 65.45 0.00993
63 7 65.46 0.00905
64 7 65.47 0.00825
65 7 65.48 0.00751
66 7 65.48 0.00685
67 7 65.49 0.00624
68 7 65.49 0.00568
69 7 65.50 0.00518
70 7 65.50 0.00472
71 7 65.50 0.00430
72 7 65.50 0.00392
73 7 65.51 0.00357
74 7 65.51 0.00325
75 7 65.51 0.00296
76 7 65.51 0.00270
77 7 65.51 0.00246
78 7 65.51 0.00224
79 7 65.51 0.00204Lastly, we can use cross-validation (see Section 8.8.1) to choose an optimal cv.glmnet function:
cvfit.lasso <- cv.glmnet(x = x,
y= mammalsc$total_sleep, alpha=1)We can use the associated plot function to see how our estimates of mean-squared error change with
plot(cvfit.lasso)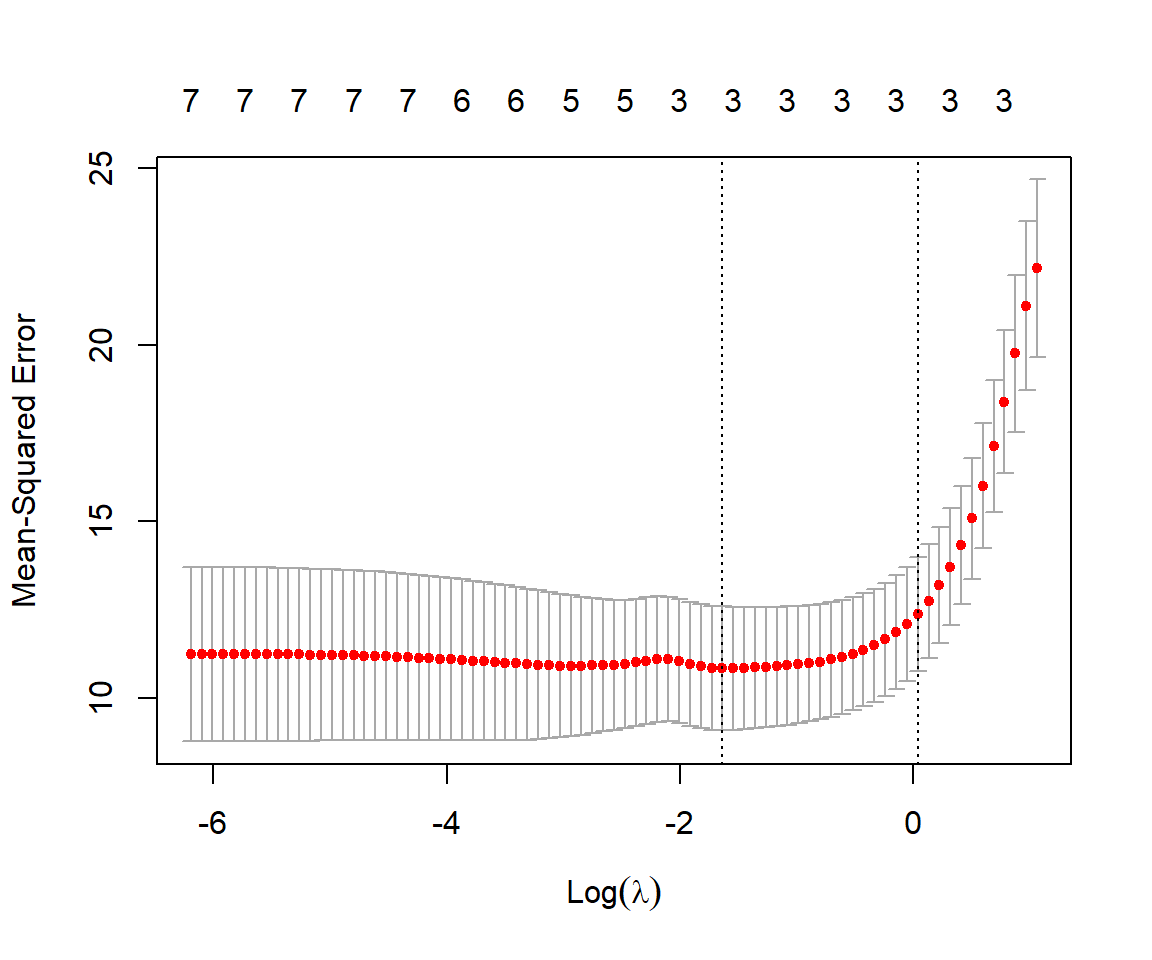
For each value of
cvfit.lasso$lambda.min[1] 0.1949944coef(cvfit.lasso, s="lambda.min")8 x 1 sparse Matrix of class "dgCMatrix"
s1
(Intercept) 16.976166754
life_span .
gestation -0.008038413
logbrain_wt -0.548365524
logbody_wt .
predation .
exposure .
danger -1.398245832We see that we are left with the same 3 predictors as the model with the lowest
allsubsets[1,]Global model call: lm(formula = total_sleep ~ life_span + gestation + log(brain_wt) +
log(body_wt) + predation + exposure + danger, data = mammalsc)
---
Model selection table
(Int) dng log(brn_wt) prd df logLik AICc delta weight
98 16.4 -3.628 -0.6763 1.992 5 -103.722 219.1 0 1
Models ranked by AICc(x) We can repeat the process using Ridge regression:
cvfit.ridge <- cv.glmnet(x = x,
y= mammalsc$total_sleep, alpha=0)
cvfit.ridge$lambda.min[1] 1.408017coef(cvfit.ridge, s="lambda.min")8 x 1 sparse Matrix of class "dgCMatrix"
s1
(Intercept) 16.225126140
life_span -0.008771936
gestation -0.007109805
logbrain_wt -0.314594127
logbody_wt -0.181114581
predation -0.193136878
exposure -0.145969577
danger -0.948772311In this case, all coefficients are non-zero. Lastly, if we compare these coefficients to those from model-averaging and from the full model (Figure 8.5), we see that the coefficients from Ridge regression are shrunk towards 0, in this case more so than model averaging.
One challenge with penalization-based methods is that it is not straightforward to calculate appropriate confidence intervals due to the bias introduced by the penalization, and therefore glmnet does not supply SEs. In many ways, a Bayesian treatment of regularization seems more natural in that the penalization terms can be viewed as arising from specific prior specifications (Tibshirani 2011; Casella et al. 2010). For a fuller treatment of regularization methods and connections between the LASSO, Ridge Regression, and Bayesian methods, see Hooten and Hobbs (2015).
8.8 Evaluating model performance
We have now seen a few different modeling strategies, identified some of the challenges inherent to them, including the potential of overfitting data, and discussed ways that we can improve predictive performance via shrinkage of coefficients towards 0. It is also important that we have tools for evaluating models and modeling strategies. Two powerful approaches are cross-validation, which was briefly mentioned in the previous section, and bootstrapping.
8.8.1 Cross-validation
One potential issue with evaluating models via data splitting (i.e., the approach I used during my Linear Regression midterm; Section 8.3) is that estimates of model performance can be highly variable depending on how observations get partitioned into training and test data sets. One way to improve upon this approach is to use
- Split the data into many subsets (
- Loop over
- Fit the model using data from all subsets except
- Predict the response for data in the
- Fit the model using data from all subsets except
- Pool results and evaluate model performance, e.g., by comparing
We will demonstrate this process using functions in the caret package (Kuhn 2021), though it is also easy to implement the approach on your own (e.g., using a for loop). The caret package allows one to implement various forms of data partitioning/resampling via its trainControl function. Here, we will specify that we want to evaluate the performance using cross-validation (method = "cv") with 10 folds (number = 10). Let’s evaluate the model containing our same set of sleep predictors.
library(caret)
set.seed(1045) # to ensure we get the same result every time.
# defining training control
# as cross-validation and
# value of K equal to 10
train_control <- trainControl(method = "cv",
number = 10)
# training the model by assigning total_sleep column
# as target variable and rest other column
# as independent variable
model <- train(total_sleep ~ life_span + gestation + log(brain_wt) +
log(body_wt) + predation + exposure + danger,
data = mammalsc,
method = "lm",
trControl = train_control)
# printing model performance metrics
# along with other details
print(model)Linear Regression
42 samples
7 predictor
No pre-processing
Resampling: Cross-Validated (10 fold)
Summary of sample sizes: 36, 38, 38, 38, 38, 38, ...
Resampling results:
RMSE Rsquared MAE
3.598593 0.5286154 3.001091
Tuning parameter 'intercept' was held constant at a value of TRUEWe can repeat this approach multiple times (referred to as repeated k-fold cross validation) by changing the method argument of trainControl to repeatedcv and by specifying how many times we want to repeat the process using the repeats argument.
train_control <- trainControl(method = "repeatedcv",
number = 10, repeats=5)
# training the model by assigning sales column
# as target variable and rest other column
# as independent variable
model <- train(total_sleep ~ life_span + gestation + log(brain_wt) +
log(body_wt) + predation + exposure + danger,
data = mammalsc,
method = "lm",
trControl = train_control)
# printing model performance metrics
# along with other details
print(model)Linear Regression
42 samples
7 predictor
No pre-processing
Resampling: Cross-Validated (10 fold, repeated 5 times)
Summary of sample sizes: 37, 38, 38, 39, 38, 36, ...
Resampling results:
RMSE Rsquared MAE
3.328851 0.6342511 2.792544
Tuning parameter 'intercept' was held constant at a value of TRUEWe can compare these results to the estimate
summary(fullmodel)$r.squared[1] 0.6551552summary(fullmodel)$sigma[1] 3.036907We see that the cross-validation estimate of lm. Although the differences are small here, cross-validation can be particularly useful for estimating out-of-sample performance (i.e., performance of the model when applied to new data not used to fit the model) when model assumptions do not hold.
Most ecological data sets are subjected to some form of correlation due to repeated observations on the same sample unit or spatial or temporal autocorrelation, and thus, observations will not be independent. When applying cross-validation to data with various forms of dependencies, it is important to create folds that are independent of each other (Roberts et al. 2017); the data within folds, however, can be non-independent. For spatial or temporal data, this can often be accomplished by creating blocks of data that are clustered in space or time. Several R packages have been developed to facilitate spatial cross-validation, including sperrorest (Brenning 2012), ENMeval (Kass et al. 2021), and blockCV (Valavi et al. 2019).
8.8.2 Boostrapping to evaluate model stability
So far, we have looked at how we can evaluate model performance using cross-validation. This process attempts to evaluate model performance when applied to a new data set. Our examples in the last section assumed we had a pre-specified model. What if we wanted to also evaluate the impact of various modeling choices along the way – e.g., stepwise-selection procedures or tuning parameters (e.g., rms package (Harrell Jr 2021).
To evaluate model performance using a bootstrap, we will mimic the entire process from start to finish, including:
- Creating training and test data sets using separate bootstraps.
- Training the model using the training data (this step could include choosing tuning parameters or using stepwise-selection algorithms to determine a final model).
- Evaluating the model using the test data.
To estimate model performance metrics (Figure 8.6 A), we begin by estimating the average degree of optimism resulting from evaluating the model using the same data as was used to train the model. We then subtract this optimism from the same performance metrics calculated using the full data set. The steps here are:
- Fit a model to the full data set and estimate
- Form bootstrapped test and bootstrapped training data sets by resampling the original data set with replacement.
- Fit the full model to the bootstrapped training data set and estimate
- Use the model from step 3 to form predictions for the test data set.
- Use the predictions from step 4 and the test data to estimate
- Estimate the degree of optimism,
- Estimate an improved
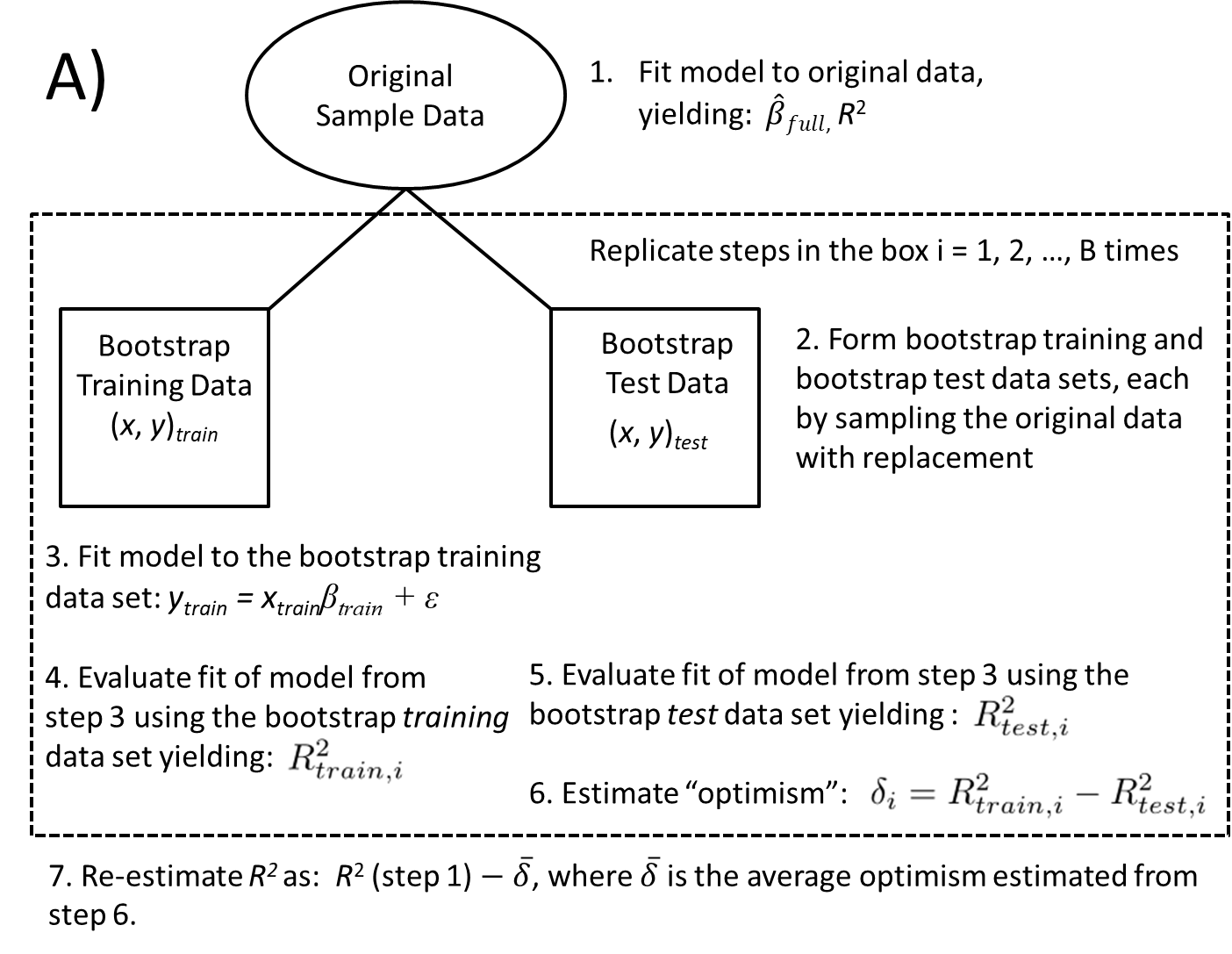
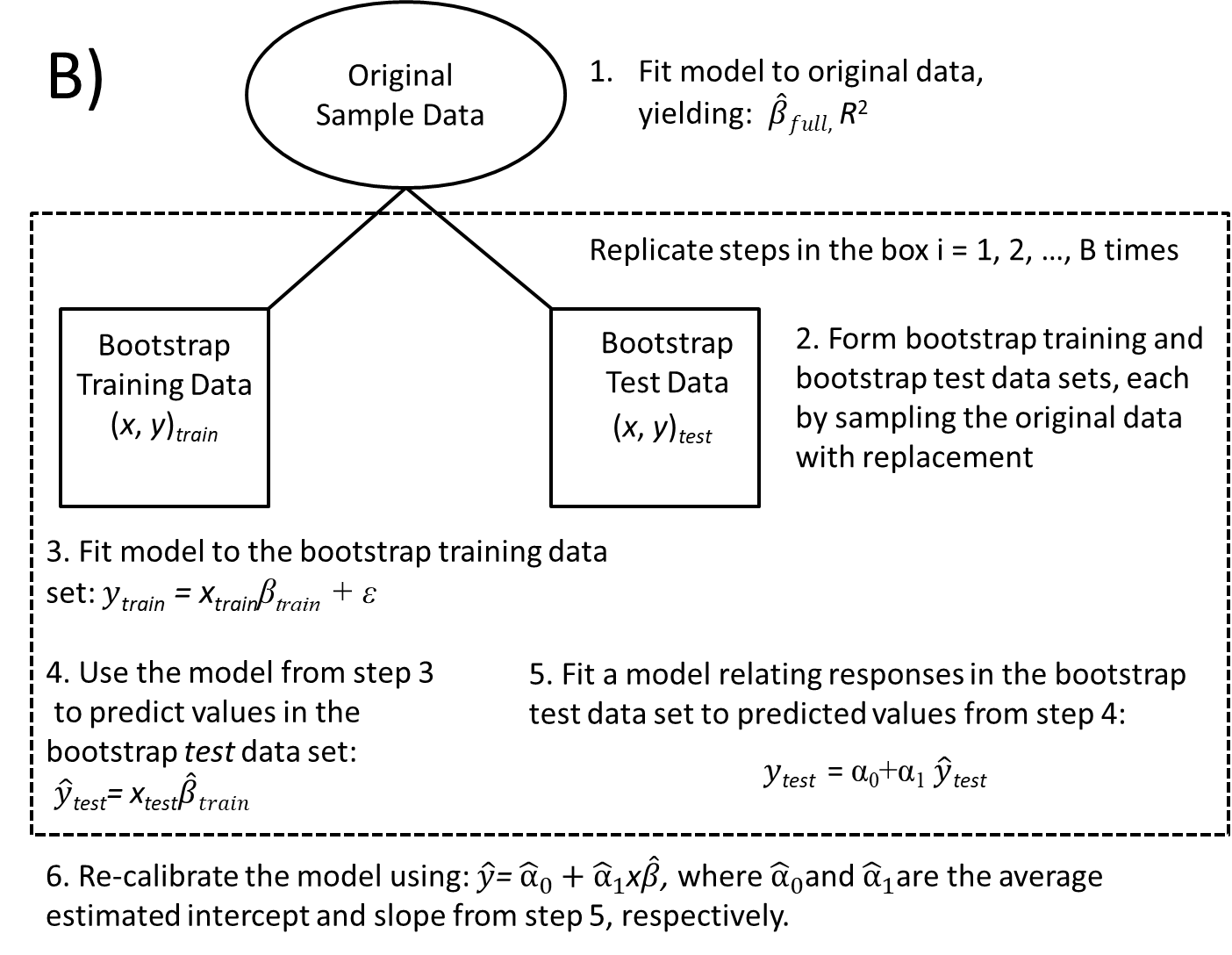
We can also use a bootstrap to recalibrate our predictions. If we have overfit the data, it may help to shrink our predictions back towards the mean response in the data set (Figure 8.6 B). We can estimate an appropriate degree of shrinkage by comparing how well our predictions from models fit to the training data match observations in the test data set. Here the steps are similar to those outlined above:
- Estimate regression parameters using the full data set.
- Form bootstrapped test and bootstrapped training data sets by resampling the original data set with replacement.
- Fit the full model to the bootstrapped training data set
- Use the model from step 3 to form predicted responses for the bootstrapped test data set.
- Fit a linear regression model using the test data responses (as the response variable) and the predicted values from step 4 as the only explanatory variable.
- Repeat steps 2-5 many times. The average slope in step 5 can be used to proportionally reduce, or shrink, the regression parameters from step 1 towards 0; if no overfitting has occurred, the average slope in step 5 will be 1.
The rms package has functions for evaluating a variety of models (see below), including those obtained by backwards selection. However, users must fit their models using functions specific to the rms package:
- linear regression models (using
olsrather thanlm) - generalized least squares (using
Glsrather thangls) - logistic regression models (using
lrmrather thanglm) - cox proportional hazards models (using
cpmrather thancph)
Unfortunately, methods for validating mixed effect models see 18 are not available in the rms package.
Let’s explore this approach with the sleep data. We must first fit the model using ols. We can then evaluate model performance when also including backwards selection using the validate function with the argument bw = TRUE.
library(rms)
set.seed(130)
fullmod.ols<-ols(total_sleep ~ life_span + gestation + log(brain_wt) +
log(body_wt) + predation + exposure + danger,
data = mammalsc, x = TRUE, y = TRUE)
validate(fullmod.ols, bw = TRUE)
Backwards Step-down - Original Model
Deleted Chi-Sq d.f. P Residual d.f. P AIC R2
body_wt 0.04 1 0.8326 0.04 1 0.8326 -1.96 0.655
life_span 0.11 1 0.7448 0.15 2 0.9275 -3.85 0.654
gestation 1.32 1 0.2500 1.47 3 0.6883 -4.53 0.640
exposure 1.76 1 0.1845 3.23 4 0.5193 -4.77 0.622
predation 3.66 1 0.0557 6.90 5 0.2284 -3.10 0.585
Approximate Estimates after Deleting Factors
Coef S.E. Wald Z P
Intercept 17.4416 1.0680 16.331 0.000e+00
brain_wt -0.9194 0.1974 -4.658 3.199e-06
danger -1.5755 0.3566 -4.418 9.977e-06
Factors in Final Model
[1] brain_wt danger index.orig training test optimism index.corrected n
R-square 0.5852 0.6279 0.5205 0.1074 0.4778 40
MSE 8.9805 7.2317 10.3815 -3.1498 12.1303 40
g 4.1115 4.0430 3.9206 0.1223 3.9892 40
Intercept 0.0000 0.0000 0.6651 -0.6651 0.6651 40
Slope 1.0000 1.0000 0.9432 0.0568 0.9432 40
Factors Retained in Backwards Elimination
life_span gestation brain_wt body_wt predation exposure danger
* * * *
* *
* * *
* *
* *
* * * * *
*
* *
* * *
* * *
* *
* *
* *
* * * *
* *
* *
* *
* * *
* *
* *
* *
* * *
*
* * *
* *
* *
* * *
* *
* *
* *
* *
* * * *
* * *
* * *
* * *
* *
* *
* *
* *
* * * * *
Frequencies of Numbers of Factors Retained
1 2 3 4 5
2 23 10 3 2 We see that backwards elimination results in a fair degree of optimism. Correcting for it decreases our estimate of validate function also shows us which predictors are chosen when the backwards selection algorithm is applied to each bootstrap sample. We see that there is quite a bit of variability in the final model that is chosen. Thus, if we were to collect another data set of the same size and apply the same model-selection algorithm, we might vary well end up with a different set of variables in the reduced model. We may choose to communicate this uncertainty due to model selection by reporting bootstrap inclusion frequencies (how often variables are selected in final models) or by listing other “competitive models” (i.e, those models that are frequently chosen across the bootstrap replicates) (Heinze, Wallisch, and Dunkler 2018).
8.9 Summary
In this section, we have seen several modeling strategies, including:
- Stepwise selection algorithms (possibly with change-in-estimate criterion)
- Full model inference (df spending)
- Model averaging (using multiple models for inference)
- Penalized estimation methods (LASSO, Ridge regression)
For describing associations, we may consider any of these methods including the use of a full model or stepwise-selection algorithms. Note, however, that interpretation of coefficients can be more challenging when using model-averaging or regularization/penalization due to the bias introduced when estimating regression coefficients with shrinkage. These latter methods are geared towards improving predictions. For inference, we are likely best off fitting a small number of informed models (e.g., representing different causal mechanisms) which we can then compare. Lastly, we have seen how cross-validation and bootstrap resampling methods can help us quantify how (possibly tuned) models will likely perform when applied to new data. There is tremendous demand for analysts that can develop good predictive models, and this has fueled the development of open-source software for tuning and evaluating models. In addition to the tools available in the caret package (Kuhn 2021), the tidymodels and workflows packages offer powerful options for evaluating models using resampling-based techniques (Kuhn and Wickham 2020; Vaughan 2021). Useful tutorials for these packages can be found here and here.
8.10 References
Albumin is sometimes used as an indicator of kidney disease.↩︎
The overall type I error is the probability of rejecting one or more null hypotheses when all of them are true↩︎
The likelihood ratio test statistic for two models that differ by a single parameter = -2(logL
1-pchisq(2, df = 1).↩︎If all of our assumptions of linear regression hold, then least squares will give us the Maximum Likelihood estimate - see Section 10.11.↩︎